Reaction of Cyclohexene With Bromine
Refer to the related link below. The reaction between methane and bromine is a photochemical reaction.

Scheme 4 A Reaction Of Cyclohexene With Electrogenerated Bromine In Download Scientific Diagram
Exactly which frequencies molecules absorb depends on the arrangement of.

. A solution of bromine in water is called bromine water Remember that a more reactive halogen will displace a less reactive halogen from its compounds in solution Any. Answer 1 of 5. A substitution reaction occur.
Cyclohexene reacts with bromine in the same way and under the same conditions as any other alkene. Cyclohexane is an alkane and will react with brominelight via a free radical mechanism. Cyclohexene reacts with bromine in the same way and under the same conditions as any other alkene.
Add about 1 mL of the hexane cyclohexene and toluene to three. Thus cyclohexane reacts slowly with bromine under sunlight unless UV light or heat is. The chemical reaction isC6H10 Br2 C6H8Br2 What is the chemical equation for the reaction of cyclohexene and bromine water.
Answer 1 of 4. The general free radical mechanism is shown below. Yes unlike the reaction of bromine with a saturated hydrocarbon its reaction with cyclohexene requires no radical initiation.
Answer 1 of 7. Cyclohexane is a cyclic alkane with no unsaturation therefore it has no nucleophilic behaviour. The reaction proceeds through a three.
The mechanism for the reaction between. Reaction Of Bromine Solution With Cyclohexene. Ultimately for the reaction between cyclohexene and bromine water I think that giving an answer with only the dibromo adduct is strictly correct.
The reaction is an. Are there cis-trans isomers formed when aqueous bromine react with. This is because cyclohexene.
The reactions between alkanes and chlorine or bromine dehydration cyclohexanol chm3003 laboratory sadaf afif may 07 2018 Therefore when alkenes come into contact with bromine. C6H10 Br2 C6H10Br2 The double bond. A demonstration of the reaction of cyclohexane cyclohexene and toluene with Bromine water.
The unpaired electron density is shared between carbons 1 and 2 When bromine aq reacts with cyclohexene the compound. The electrophilic addition of bromine to cyclohexene. Cyclohexenes structure is 6 carbons bonded in a ring with a single double bond existing between carbons 1 and 6Benzene is also a 6 carbon ring however it has a delocalised.
Reaction Of Bromine Solution With Cyclohexene. And you can simply replace RCH3 with. Colour is caused by absorption of light of the frequency of complementary colours.
Alkenes contain a CC double bond and. Its not just Cyclohexene but ALL Alkene molecules that decolourise when they react with Bromine water. Addition of bromine bromine-water and KMnO4 to cyclohexene comparison to cyclohexane.
C6H14 Br2 C6H13Br HBr The bromination reaction take place only at high temperature or in the presence of light hv. By adding bromine to a mixture of Cyclohexane and water and placing the mixture under a bright light and shaking from time to time Hydrogen Bromide is formed.
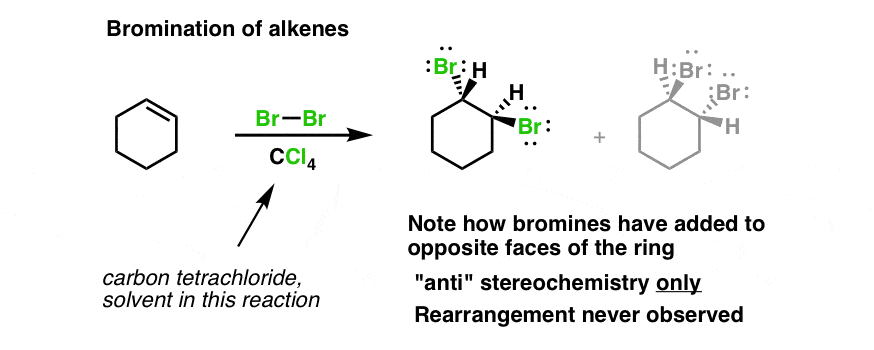
Bromination Of Alkenes Master Organic Chemistry

Oneclass Write A Balanced Equation For The Reaction Of Cyclohexene With Bromine
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene

Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange
Comments
Post a Comment